Welding of austenitic stainless steel – Part 1
There are a number of different types of steels that may be referred to as ‘stainless’; previous articles have considered ferritic and precipitation hardening steels for example. It is therefore advisable to be specific and to refer to the group to which the steel belongs in order to avoid confusion.
Although commonly referred to as ‘stainless steel’, the steels covered in this article should be more correctly referred to as austenitic, 18/8 or chromium-nickel stainless steels. As with the other types of stainless steels, the austenitic stainless steels are corrosion and oxidation resistant due to the presence of chromium that forms a self-healing protective film on the surface of the steel. They also have very good toughness at extremely low temperatures so are used extensively in cryogenic applications. They can be hardened and their strength increased by cold working but not by heat treatment.
They are the most easily weldable of the stainless steel family and can be welded by all welding processes, the main problems being avoidance of hot cracking and the preservation of corrosion resistance.
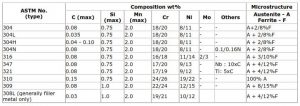
A convenient and commonly used shorthand identifying the individual alloy within the austenitic stainless steel group is the ASTM system. This uses a three digit number ‘3XX’, the ‘3’ identifying the steel as an austenitic stainless, and with additional letters to identify the composition and certain characteristics of the alloy eg type 304H, type 316L etc; this ASTM method will be used in this article.
Typical compositions of some of the alloys are given in Table 1. The type 304 grade may be regarded as the archetypal austenitic stainless steel from which the other grades are derived and changes in composition away from that of type 304 result in a change in the identification number and are highlighted in red.
The 3XX may followed by a letter that gives more information about the specific alloy as shown in the Table. ‘L’ is for a low carbon austenitic stainless steel for use in an aggressive corrosive environment ; ‘H’ for a high carbon steel with improved high temperature strength for use in creep applications; ‘N’ for a nitrogen bearing steel where a higher tensile strength than a conventional steel is required.
These suffixes are used with most of the alloy designations eg type 316L, type 316LN, type 347H, where the composition has been modified from that of the base alloy.
Austenitic stainless steels are metallurgically simple alloys. They are either 100% austenite or austenite with a small amount of ferrite (see Table 1). This is not the ferrite to be found in carbon steel but a high temperature form known as delta (δ) -ferrite. Unlike carbon and low alloy steels the austenitic stainless steels undergo no phase changes as they cool from high temperatures. They cannot therefore be quench hardened to form martensite and their mechanical properties to a great extent are unaffected by welding. Cold (hydrogen induced) cracking (Job Knowledge No. 45) is therefore not a problem and preheat is not necessary irrespective of component thickness.
Alloying elements in an austenitic stainless steel can be divided into two groups; those that promote the formation of austenite and those that favour the formation of ferrite. The main austenite formers are nickel, carbon, manganese and nitrogen; the important ferrite formers are chromium, silicon, molybdenum and niobium. By varying the amounts of these elements, the steel can be made to be fully austenitic or can be designed to contain a small amount of ferrite; the importance of this will be discussed later.
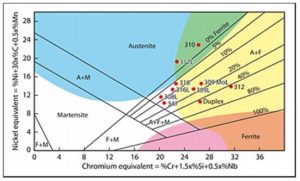
In 1949 Anton Schaeffler published a constitutional or phase diagram that illustrates the effects of composition on the microstructure. In the diagram Schaeffler assigned a factor to the various elements, the factor reflecting the strength of the effect on the formation of ferrite or austenite; these factors can be seen in the diagram.
The elements are then combined into two groups to give chromium and nickel ‘equivalents’. These form the x and y axes of the diagram and, knowing the composition of an austenitic stainless steel, enables the proportions of the phases to be determined.
Typical positions of some of the commoner alloys are given in Fig.1. Also superimposed on this diagram are coloured areas identifying some of the fabrication problems that may be encountered with austenitic stainless steels.
Although all the austenitic stainless steels are sensitive to hot cracking (see the Job Knowledge article on Solidification Cracking), the fully austenitic steels falling within the vertically blue area in Fig.1 such as type 310 are particularly sensitive.
The main culprits are sulphur and phosphorus. To this end, these tramp elements have been progressively reduced such that steels with less than 0.010% sulphur and phosphorus less than 0.020% are now readily available. Ideally a type 310 or type 317 alloy should have sulphur and phosphorus levels below some 0.003%.
Cleanliness is also most important and thorough degreasing must be carried out immediately prior to welding.
The steels such as type 304, type 316, type 347 that fall within, or close to, the small uncoloured triangular region in the centre of the diagram contain a small amount of delta-ferrite and, whilst not being immune to hot cracking, have improved resistance to the formation of sulphur-containing liquid films. The reasons for this are that a) ferrite can dissolve more sulphur and phosphorus than austenite so they are retained in solution rather than being available to form liquid films along the grain boundaries and b) the presence of quite a small amount of ferrite increases the grain boundary area such that any liquid films must spread over a greater area and can no longer form a continuous liquid film. The 100% austenitic steels do not have this advantage.
One problem that has arisen with very low sulphur steels is a phenomenon known as ‘cast to cast variation’ or ‘variable penetration’. The weld pool in a low sulphur steel (<0.005%) tends to be wide with shallow penetration; a steel with sulphur over some 0.010% has a narrower, more deeply penetrating weld bead.
This is generally only a problem with the use of the fully automated TIG welding process, a manual welder being capable of coping with the variations in penetration due to the differences in sulphur content in different casts of steel. However, automated TIG welding procedures developed on a ‘high’ sulphur steel, when used to weld a low sulphur steel may result in lack of penetration type defects; the reverse situation may result in excessive penetration. Changes to the procedure that have mitigated, but never eliminated, this problem have included slow travel speed, pulsed current, use of Ar/H2 shield gas mixtures.
Other methods include specifying a minimum sulphur of, say, 0.010% or segregating the steels into batches with known penetration characteristics and developing welding procedures to suit. The A-TIG activated flux process has also been found to be of benefit.
The problems of welding the fully ferritic steels that fall into the pink area, where grain growth and embrittlement is a problem, have already been dealt with in Job Knowledge – Welding of ferritic/martensitic stainless steels.
The austenitic stainless steels falling into the yellow area will also embrittle but this is as a result of the formation of hard brittle phases called ‘sigma’ (σ) and ‘chi’ (χ). This embrittlement takes place in the temperature range of approximately 500 to 900°C. It is a sluggish process and is not a problem during welding of the austenitic stainless steels, but can occur in elevated temperature service or if the welded component is stress relieved.