Precipitation or age hardening
There are several methods that may be used to increase the strength of a metal; alloying, quenching of steel, work hardening, and one very specific form of heat treatment, that of precipitation or age hardening (the two terms are synonymous). Many ferrous and non-ferrous alloys are capable of being age hardened and, as the name suggests, this method of increasing the strength relies upon the formation of precipitates. To achieve the optimum combination of mechanical properties the heat treatment cycles must be very closely controlled. Unfortunately, to understand how the precipitates affect the mechanical properties it is necessary to introduce some fundamental metallurgy.
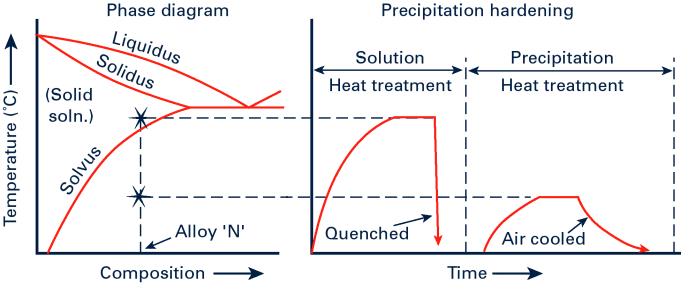
The precipitation hardening mechanism requires the solubility of the alloying element, the solute, in the metal, the solvent, to increase as the temperature increases as shown in the phase diagram in Figure 1 where the solvus line shows decreasing solubility of alloying element B in the solvent A as the temperature falls. An analogy is that of salt in water; as the temperature increases more salt can be dissolved but the converse happens as the solution is allowed to cool when salt crystals begin to form or precipitate.
The same process occurs in suitably alloyed metals except that the processes of dissolving and precipitating take place in the solid and are hence much slower as atoms find it more difficult to move in a solid than a liquid solution.
A consequence of this is that once the precipitates have been dissolved by taking the metal alloy to a sufficiently high temperature, ie above the solvus line, they can be prevented from re-forming by rapid cooling or quenching.
This heat treatment is known as solution heat treatment and is carried out to form an unstable super-cooled solid solution which, if reheated to a lower ageing or precipitation hardening temperature, will begin to re-form the precipitates, these growing in size as the heat treatment proceeds. A schematic of such a heat treatment cycle is also given in Figure 1 for alloy N.
In the solution heat treated metal the atoms of the alloying element, the solute, are randomly distributed throughout the matrix but once the temperature is raised the precipitates begin to form by a nucleation and growth process. At relatively low temperatures and in a short timescale the solute atoms begin to cluster together to form extremely small and very finely dispersed precipitates known as Guinier-Preston (GP) zones, named after the two metallurgists who first identified them. The GP zones are so small that they are not visible using normal optical microscopes but can be seen using electron microscopy at magnifications of around x100,000.
The GP zones are described as coherent, in other words they have the same crystal structure as the solvent metal. However, they distort the crystal lattice, the framework on which the atoms are positioned. This makes it more difficult for dislocations to move through the lattice and it is dislocation movement that enables metal to deform; tensile strength and hardness therefore increase but ductility and toughness decrease.
As the ageing treatment continues or the temperature is raised the tensile strength also continues to increase as the precipitates grow and coarsen whilst still remaining coherent. At some point, however, the precipitates begin to lose their coherency; they become incoherent, forming separate particles within the metal with a different crystal structure from the solvent and at this stage they become visible using an optical microscope.
Just before this point is reached is when the alloy has the very highest tensile strength. As these incoherent particles form and grow in size the tensile strength progressively decreases. The alloy then is said to be overaged although the precipitates still contribute towards the tensile strength of the alloy. The high strength low alloy (HSLA) steels are a good example of this where incoherent, overaged precipitates are used to give a substantial increase in the tensile strength.
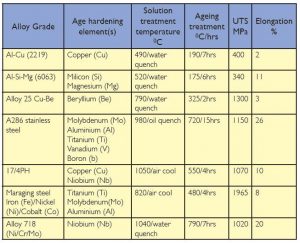
In order to achieve the best combination of properties the precipitates need to be evenly distributed throughout the grains of the alloy and of an optimum size. The ageing temperature and/or time can obviously be changed to tailor the distribution and size of the precipitates; longer times and/or higher temperatures generally result in a reduction in strength but an increase in ductility, an overaged structure giving the lowest tensile strength but the highest ductility. Typical heat treatment times and temperatures of a range of different alloys are given in Table 1. The ferritic and nickel based alloys are generally used in the overaged condition in order to ensure a reasonable degree of ductility. It can be seen that with some alloys, eg 17/4PH stainless steel, the precipitation mechanism is sufficiently sluggish that the component can be cooled in still air or, as with the A286 stainless steel, long ageing times are required.
On the other hand the aluminium-copper alloy 2219 is capable of ageing at room temperature if left for a couple of days. Some of the 6000(Al-Si-Mg) and series 7000 (Al-Zn-Mg) alloys will similarly age at ambient temperature. This is known as natural ageing; – aging at an elevated temperature is known as artificial ageing.
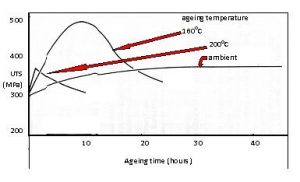
The close control of heat treatment times, temperatures and cooling rates is therefore essential if the required properties are to be obtained. For the solution treatment of aluminium alloys a salt bath is frequently used, artificial ageing taking place in a forced air circulation furnace. Illustrated in Figure 2 is the effect of varying the time and temperature on the ultimate tensile strength of an Al-4%Cu alloy such as alloy 2025 where it can be seen that a difference as small as 40OC in the ageing temperature can have a major effect on the strength. The higher temperature needed by the nickel and ferrous alloys generally requires the use of gas fired or electrical furnaces with sufficient thermocouples to ensure the correct temperatures are consistently achieved throughout the component.
This article was written by Gene Mathers.
Copyright © TWI Ltd 2014
The content of this article was correct at the time of publication. For more information visit www.twi-global.com